The medical industry is one of the fastest growing segments of any economy. Increasingly,
Medical equipment is being used outside a controlled hospital environment. The complexity and increased use of medical equipment in non-hospital environments has made the need for safe, reliable products imperative. With the advent of Technology and stringent regulations, the need for a Reliability and Excellence has never been more vital.
With the extensive knowledge on medical device Products and application of Reliability and Efficiency programs we are well equipped to support the industry have a strong presence in the global arena.
Our Service Offering comprises of the following,
Reliability Engineering Services
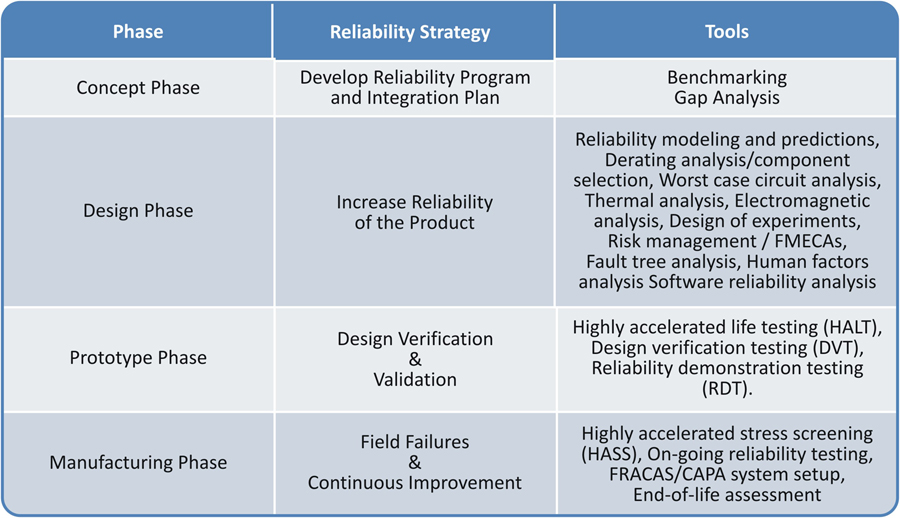
Reliability Testing
Reliability Development / Growth Test (RD / GT)
– MTBF or MTTF Tests
Reliability Qualification Tests (RQT),
– Vibration , Shock , Temperature Test
Performance Tests, Screening Tests
Probability Ratio Sequential Tests (PRST).
Standards and Regulatory services
– ISO 13485
– FDA 21 CFR Part 820
– IEC 60601-1 standards
– ISO 9000 Series
– IEC 605 – Equipment Reliability Testing
Process Improvement, Efficiency Projects and Training
o Quality Engineer
o Quality Auditor
o Quality Process Analyst
o Root Cause Analysis & Corrective Action
o Lean Manufacturing
o Lean Six Sigma – Black Belt / Green Belt / Master Black Belt
o 7QC Tools and Advance QC Tools
o Statistical Process Control (SPC)
o Problem Solving Tools
o 8 D Analysis
o Quality (ISO 9001)
o Information Security (ISO 27001)
o Environment (ISO 14001)
o Occupational Health & Safety (OHSAS 18001)
o Internal Auditor Training
o Auditor / Lead Auditor Training
o Advanced RAMS program
o Reliability Engineer (CRE)
o RAMS training
o Risk management
o Hazard analysis
o RAMS analysis
o RAMS tests
o Life Cycle Cost (LCC) analysis
o Common Safety Methods (CSM)
o Functional Safety Certification Program
o Safety Management System (SMS)Risk Analysis
o Software Reliability Engineer
o Software Safety Requirement Specification (SW-SRS)
o Software – Life cycle, architectures, firmware and user SW